My research investigates how temperature and other environmental factors shape transmission of infectious diseases, with a current focus on mosquito-borne diseases.
My work combines field data, laboratory experiments, and mathematical models. I connect phenomena across biological scales by characterizing the community and organism-level mechanisms that generate population-level infection dynamics across temporal and spatial scales, including seasonality, interannual variation, and geographic distributions.
Thermal disease ecology
Temperature is one of the most fundamental forces shaping life on earth. However, predicting the impact of temperature—and future climate change—on transmission of infectious disease is challenging for several reasons:
- The thermal responses of the host and parasite traits that drive transmission are typically nonlinear and often unimodal.
- Transmission itself is a nonlinear process incorporating multiple host and parasite traits that may have opposing thermal responses.
- Thermal responses vary among host and parasite species, and most disease transmission occurs in the context of complex ecological communities.
- Temperature varies at multiple relevant temporal and spatial scales.
- Temperature often covaries with other environmental drivers.
Consequently, it can be difficult to causally link variation in temperature to observed variation in disease and to predict how changes in temperature will affect transmission. This is especially true when climate change means that future conditions may exceed currently observed temperature ranges or de-couple associations between co-varying drivers.
My research uses trait-based approaches to address these challenges.
Mosquito-borne disease
As a postdoc, I investigated how thermal performance varies across mosquito and pathogen species and how these differences affect the predicted thermal response of transmission. I led studies on Ross River virus (Shocket et al. 2018 eLife, PDF), the most common vector-borne disease in Australia, and on West Nile virus and a suite of five other viruses with overlapping vector species (Shocket et al. 2020 eLife, PDF).
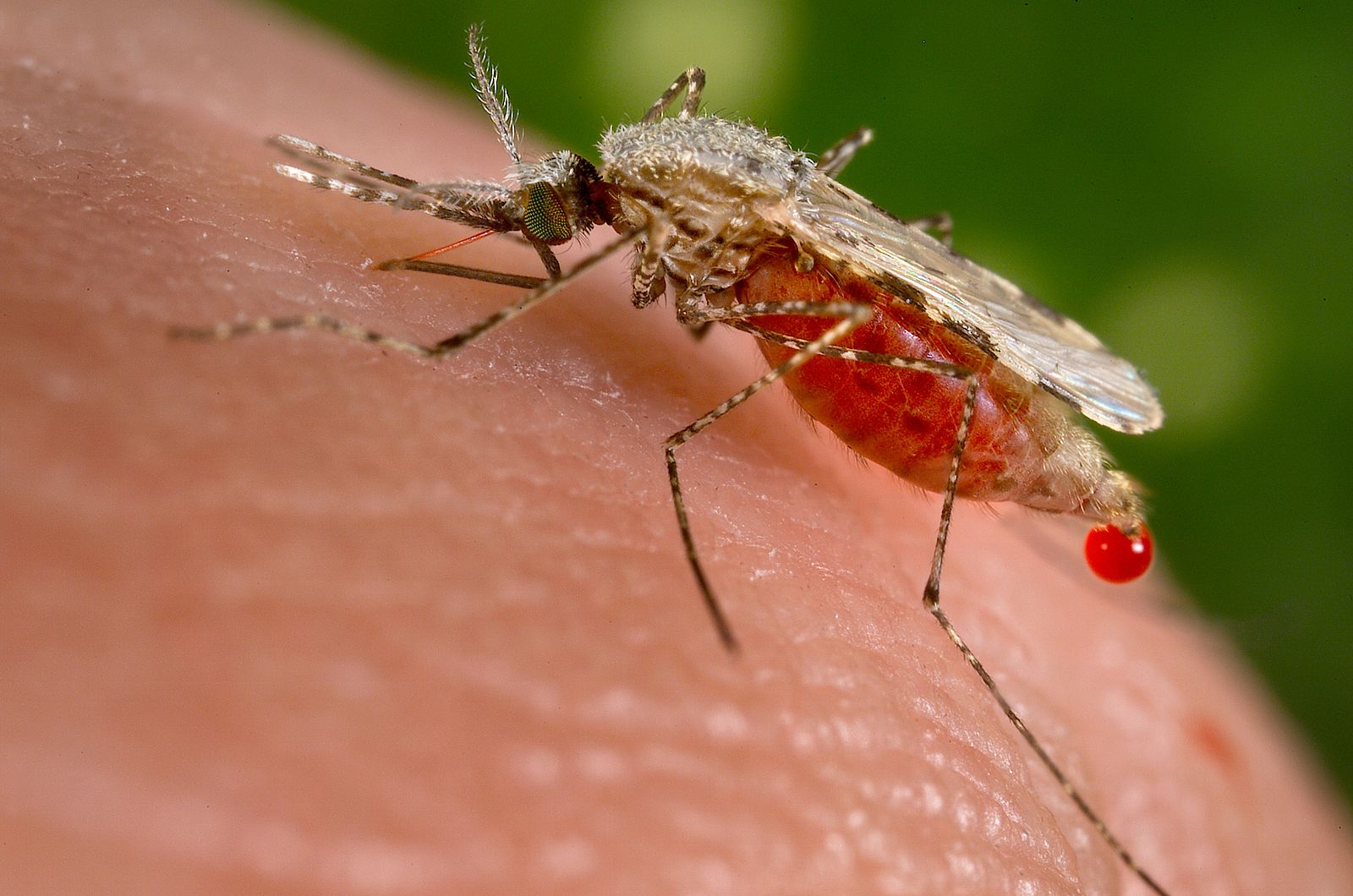
Together with studies led by my colleagues studying the malaria parasite (Mordecai et al. 2013 Eco Lett, Johnson et al. 2015 Ecology), dengue virus (Mordecai et al. 2017 PLOS NTD, PDF), and Zika virus (Tesla et al. Proc B), we have generated a systematic analysis of 16 different mosquito-pathogen systems using common methods (Mordecai et al. 2019 Eco Lett, PDF). This approach allows us to make direct comparisons between different vector-pathogen systems.
We found substantial variation in the thermal responses of vector and pathogen species that led to differences in predicted transmission (Mordecai et al. 2019 Eco Lett, PDF). Nonetheless, transmission for all vector-pathogen pairs is optimized at an intermediate temperature - a “goldilocks temperature” that is neither too hot nor too cold - in the relevant temperature range in nature. These findings contrast with many studies that overestimate the optimum and instead support the outdated “warmer-is-(always)-sicker” hypothesis.
The results from these transmission models predict seasonal and spatial variation in human cases, evidence that temperature casually drives transmission (Shocket et al. 2018 eLife, PDF; Shocket et al. 2020 eLife, PDF). Additionally, my analysis of West Nile virus encephalitis cases provides some of the strongest evidence to date that hot temperatures already constrain transmission across portions of the U.S., and transmission is likely to decrease with climate change in many areas (Shocket et al. 2020 eLife, PDF).
In general, our results indicate that the impacts of climate change on mosquito-borne disease will vary by location and time of year, sometimes increasing transmission and sometimes decreasing it. Climate change may also shift which diseases predominate in specific locations. For instance, Africa could see a shift from malaria to arboviruses (Mordecai et al. 2020 Lancet Planetary Health).
Most recently, I worked on a project measuring the impact of daily temperature variation on mosquito performance in Anopheles stephensi and testing mathematical methods for predicting the impact of this type of variation (Shocket et al. 2025 Nature Communications).
Daphnia-fungal pathogen
For my PhD, I used a Daphnia-fungal pathogen system to test the hypothesis that warmer temperatures promoted larger epidemics, as suggested by several years of field data.

I conducted lab experiments to measure how temperature affected host and parasite traits and used these thermal responses to parameterize mathematical models for transmission. This analysis revealed that warmer temperatures increase the foraging rate of hosts (and contact with parasites, which they encounter while feeding) and increase the parasite’s ability to successfully infect hosts (Shocket et al. 2018 Am Nat, PDF).
Surprisingly, the parasite’s ability to infect hosts depended primarily on the temperature at which it was produced (i.e., the temperature of the previous infection), rather than on the temperature in which it encountered and infected new hosts (Shocket et al. 2018 Ecology, PDF). This “trans-host” effect is a novel biological mechanism by which temperature can drive infection dynamics. It could also be important in other host-parasite systems, although we have very little data because it is so understudied.
I also tested another hypothesis motivated by field observations: do extreme hot temperatures exclude epidemics during the summer? Hot temperatures did reduce transmission via the trans-host effect and other damage to parasites. However, these effects were not strong enough to explain the lack of summer epidemics (Shocket et al. 2019 Func Ecol, PDF).
Ongoing Research
I have several research projects extending my previous work on mosquito-borne disease, including:
-
testing how mathematical methods for incorporating diurnal temperature variation affect the accuracy of transmission models using observed cases of arboviruses (collaboration with Courtney Murdock and Laura Multini at Cornell University)
-
parameterizing a transmission model for avian malaria (collaboration with Oswaldo Villena and colleagues at University of Hawai’i)
-
making spatially explicit predictions for the impact of climate change on transmission of Ross River virus in Australia and West Nile virus in the US (with Sadie Ryan at University of Florida)
Daphnia photo credit: Dr. Meghan Duffy (University of Michigan)
